Group Markkanen

Cancer as a global healthcare challenge
The term cancer comprises a large group of complex diseases that is responsible for an estimated 10 million deaths, or about 1 in 6 deaths, in 2020 world-wide[1]. As the second most frequent cause of death both in Europe and worldwide, it causes significant socio-economic impact. In Switzerland, more than 45’000 people are newly diagnosed with cancer each year and represents the most frequent cause for premature mortality[2]. Due to the increasing average age of the general population, these numbers are predicted to rise further in the near future. Increased understanding of the molecular underpinnings of these devastating diseases is required to develop better treatment options for affected patients.
Tumours arise when cells start to divide uncontrollably and stop obeying the architectural rules of the tissue. While the vast majority of research in the past has focused on the cancer cells themselves, recent progress has started to unveil the importance of the stromal tissue environment in cancer formation and progression. The so-called tumour stroma consists of a heterogeneous mixture of extracellular matrix as well as a multitude of cells, including angiogenic vascular cells, immune cells and fibroblasts. Under physiological conditions, by maintaining epithelial polarity, stroma serves as an important barrier to prevent epithelial transformation. However, in response to emerging epithelial cancerous lesions, the stromal compartment undergoes a reprogramming towards a tumour-supportive function, termed cancer-associated stroma (CAS). CAS has been abundantly shown to play a key role in initiation, progression and even therapy resistance of a wide variety of tumours. Despite much progress in understanding this cross-talk, many open questions remain. Improved understanding of the tumour-stroma cross-talk holds a lot of translational potential for clinical applications.
Harnessing the power of comparative oncology – “one health” in action
A plethora of in vitro and in vivo models have been used over the last century to gain insights into cancer biology. While these models have undoubtedly been highly informative in many aspects and lead to various scientific breakthroughs, the inherent limit in most of the used models is their inability to fully replicate the conditions and mirror the complexity of spontaneously developing patient tumours[3,4]. The field of comparative oncology aims to address these shortcomings by widening the research focus from classical rodent models towards spontaneous tumours that develop in other animals, such as domestic dog or cat. This additional perspective is perceived as a chance to complement and enhance our understanding of complex diseases, such as cancer, as the comparison of tumour development and risk factors across species provides the opportunity to discover basic mechanisms of tumorigenesis and develop novel therapeutic approaches to benefit both human and veterinary patients.
“ Leveraging the power of comparative oncology to improve the diagnosis and therapy of cancer in animals and humans – from patient to the bench and back“
Identifying novel diagnostic opportunities and therapeutic vulnerabilities in the cancer-stroma-crosstalk
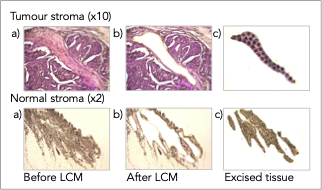
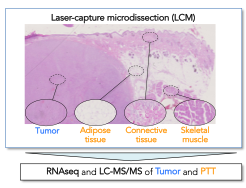
To analyze spatially defined areas of archival patient tissue, we have developed a powerful approach to isolate microscopically defined tissue areas from formalin-fixed paraffin embedded (FFPE) tumor samples using laser-capture microdissection (LCM) for analysis by RNAsequencing (RNAseq) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). This species-agnostic approach allows detailed molecular insight into patient-derived tumour samples and enables comparisons on both proteomic and transcriptomic levels to identify commonalities and differences between different tumour types across species.
Using these powerful methods to assess clinically relevant samples, we have investigated molecular mechanisms in stroma through comparative analysis of various canine and human tumors. Specifically, we have demonstrated highly conserved stromal reprogramming in canine and human mammary carcinomas and have identified disease-modulating stromal components by comparing benign and malignant canine and human mammary tumors. Further, we have investigated stromal reactions in human squamous cell carcinomas and identified disease-promoting features therein. We have also delineated the extent of molecular homology between canine spontaneous oral squamous cell carcinomas and human head-and-neck squamous cell carcinomas and identified conserved disease drivers and therapeutic vulnerabilities. The ultimate goal of these endeavours is the identification of novel diagnostic and therapeutic targets to improve care for both human and veterinary patients suffering from cancer.
Selected publications:
Ettlin J, Clementi E, Amini P, Malbon A, and Markkanen E:
Analysis of Gene Expression Signatures in Cancer-Associated Stroma from Canine Mammary Tumours Reveals Molecular Homology to Human Breast Carcinomas.
International Journal of Molecular Sciences 2017. doi: 10.3390/ijms18051101.
https://www.mdpi.com/1422-0067/18/5/1101
Amini P, Ettlin J, Opitz L, Clementi E, Malbon A, Markkanen E:
An Optimised Protocol for Isolation of RNA from Small Sections of Laser-Capture Microdissected FFPE Tissue Amenable for Next-Generation Sequencing.
BMC Molecular Biology 2017. doi: 10.1186/s12867-017-0099-7.
https://bmcmolbiol.biomedcentral.com/articles/10.1186/s12867-017-0099-7
Amini P, Nassiri S, Ettlin J, Malbon A, Markkanen E: Next-generation RNA sequencing of FFPE subsections reveals highly conserved stromal reprogramming between canine and human mammary carcinoma.
Disease Models and Mechanisms 2019. doi: 10.1242/dmm.040444.
Amini P, Nassiri S, Malbon A, Markkanen E:
Differential stromal reprogramming in benign and malignant naturally occurring canine mammary tumours identifies disease-promoting stromal components.
Scientific Reports 2020. doi: 10.1038/s41598-020-62354-8.
https://www.nature.com/articles/s41598-020-62354-8
Guscetti F, Nassiri S, Beebe E, Rito Brandao I, Graf R, Markkanen E:
Molecular homology between canine spontaneous oral squamous cell carcinomas and human head-and- neck squamous cell carcinomas reveals disease drivers and therapeutic vulnerabilities.
Neoplasia 2020. doi: 10.1016/j.neo.2020.10.003.
https://www.nature.com/articles/s41598-020-62354-8
Pöschel A, Beebe E, Kunz L, Amini P, Guscetti F, Malbon A, Markkanen E:
Identification of disease-promoting stromal components by comparative proteomic and transcriptomic profiling of canine mammary tumours using laser-capture microdissected FFPE tissue.
Neoplasia 2021. doi: 10.1016/j.neo.2021.03.001.
https://www.sciencedirect.com/science/article/pii/S1476558621000117
Beebe E, Motamed Z, Opitz L, Cheng PF, Levesque MP, Markkanen E*, Feldmeyer L*: Defining the molecular landscape of cancer-associated stroma in cutaneous squamous cell carcinoma.
Journal of Investigative Dermatology 2022. doi: 10.1016/j.jid.2022.06.017.
* joint senior authorship
https://www.sciencedirect.com/science/article/pii/S0022202X22016694?via%3Dihub
Banik I, Ghosh A, Beebe E, Burja B, Frank Bertoncelj M, Dooley CM, Markkanen E, Dummer R, Busch-Nentwich EM, and Levesque MP:
P38 mediates tumor suppression through reduced autophagy and actin cytoskeleton changes in NRAS mutant melanoma.
Cancers (Basel), 2023 Jan 31;15(3):877. doi: 10.3390/cancers15030877.
https://www.mdpi.com/2072-6694/15/3/877
Exploring the potential of soft-tissue sarcomas in dogs and cats as models for the human disease
Soft-tissue sarcomas (STS) are a heterogeneous group of rare tumors that display locally invasive growth and variable metastatic potential depending on localization and grading. While the low incidence in humans hampers detailed understanding of the disease, STS are frequent in dogs and cats and present potential models for the human condition. However, a lack of in-depth molecular characterization of STS and unaffected peritumoral tissue (PTT) in all three species impedes the translational potential of dogs and cats.
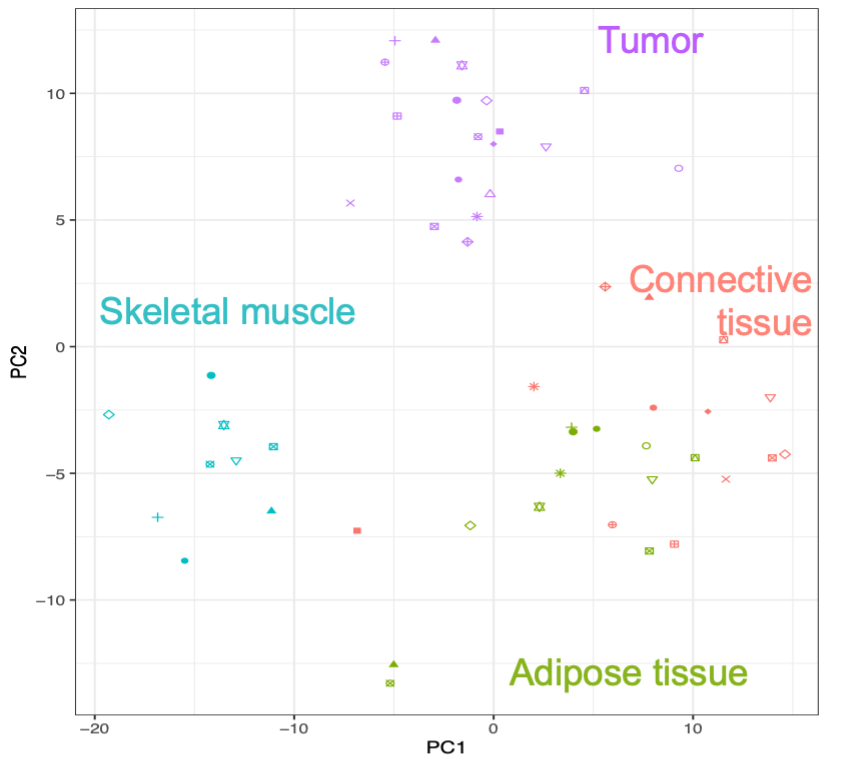
To start addressing this shortcoming, we have applied our LCM-LC-MS/MS approach to characterize the canine STS subtype Fibrosarcoma (FSA) and matched skeletal muscle, adipose and connective tissue in 30 archival samples. Our analyses provide the first detailed overview of proteomic changes in FSA and surrounding PTT with relevance for the human disorder.
Current and future work on human, feline and canine subtypes will provide highly valuable insight into these disorders in both species, inform on the cross-species homologies and differences, identify novel tumour-specific diagnostic markers and support development of novel therapies to treat these cancers.
Selected publications:
Beebe E, Pöschel A, Kunz L, Wolski W, Motamed Z, Meier D, Guscetti F, Nolff MC* and Markkanen E*: Proteomic profiling of canine fibrosarcoma and adjacent peritumoral tissue.
Neoplasia 2023. doi: 10.1016/j.neo.2022.100858.
* joint senior authorship
https://www.sciencedirect.com/science/article/pii/S1476558622000835?via%3Dihub
Addressing the effect of persistent DNA damage on cellular physiology and pathology
Genetic instability, provoked by exogenous mutagens, is well linked to initiation of cancer. However, even under physiological conditions, DNA undergoes a plethora of spontaneous alterations provoked by its inherent chemical instability and the intracellular aquatic milieu. Base excision repair (BER) is the major cellular pathway responsible for repair of these lesions, and as deficiency in BER activity results in DNA damage, which has been proposed to trigger the development of sporadic cancers. In this context, we have demonstrated that persistent DNA damage induces a cancer-cell promoting secretory phenotype in primary fibroblasts, implicating a causal role for DNA damage in the development of cancer-associated stroma. Follow-up work led us to the discovery that persistent DNA damage triggers activation of the integrated stress response to support cell survival under nutrient restriction. Furthermore, we have recently uncovered a connection between persistent DNA damage and a sex-specific anxiety-like phenotype with relevance for neuropsychiatric disorders. Further work aims at further clarifying the contribution of persistent DNA damage to the development of various pathologies.
Selected publications:
Markkanen E, Fischer R, Ledentcova M, Kessler BM, and Dianov GL:
Cells deficient in base-excision repair reveal cancer hallmarks originating from adjustments to genetic instability.
Nucleic Acids Research 2015. doi: 10.1093/nar/gkv222;
https://academic.oup.com/nar/article/43/7/3667/2414627
Legrand AJ, Poletto M, Pankova D, Clementi E, Moore J, Castro-Giner F, Ryan AJ, O’Neill E, Markkanen E*, Dianov GL*:
Persistent DNA strand breaks induce a CAF-like phenotype in normal fibroblasts.
Oncotarget 2018. doi: 10.18632/oncotarget.24446.
* joint senior authorship
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5862606/
Clementi E, Inglin L, Beebe E, Gsell C, Garajova Z, Markkanen E:
Persistent DNA damage triggers activation of the integrated stress response to promote cell survival under nutrient restriction.
BMC Biology 2020. doi: 10.1186/s12915-020-00771-x.
https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-020-00771-x
Mueller FS, Amport R, Notter T, Schalbetter SM, Lin HY, Garajova Z, Amini P, Weber-Stadlbauer U*, Markkanen E*:
Deficient DNA base-excision repair in the forebrain leads to a sex-specific anxiety-like phenotype in mice. BMC Biology 2022. doi: 10.1186/s12915-022-01377-1.
https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-022-01377-1
Arcturus Cellect LCM system
Arcturus Cellect Laser-captur microdissection system
Laser microdissection is a well-established method for isolating specific tissue areas or cell populations from tissue specimen or heterogeneous cell populations (Figure 1).
Areas of interest are identified under the microscope (using 2x, 4x, 10x, 20x, 40x, 60x objectives) and isolated using a combination of laser-capture and laser cutting with two lasers (UV and IR). The system includes LED brightfield, Phase Contrast, and LED Epi-Fluorescence Illumination.
If you are interested in using the Arcturus Cellect LCM System, please contact Enni Markkanenenni.markkanen@vetpharm.uzh.ch for details.